1. What is the more generally accepted development route for lithium batteries in the industry?
Through the unremitting efforts of R & D personnel and engineers, from lead-acid batteries, nickel-metal hydride batteries, nickel-cadmium batteries, to lithium iron phosphate batteries, and now mainstream ternary batteries, every improvement is a generation of efforts Based on improving the safety, energy density, and rate performance of lithium batteries, combined with the current status of battery research and development, a future development route for lithium batteries is summarized.

In 2020, it is a multi-cation electrode, mainly composed of NCM and NCA composite cathode materials, and the negative electrode is mainly composed of C and some silicon-carbon composites, with an energy density of about 300-350wh / kg.
From 2020 to 2025, it will be dominated by all-solid-state lithium-ion batteries with metal lithium anodes or silicon-carbon anodes. The energy density is 400wh / kg. At the same time, sodium ion batteries are developed. Sodium is cheaper than lithium, but it is larger than lithium ion, and there is liquid memory.
After 2025, the development of lithium-sulfur batteries-> lithium metal batteries->> lithium-air batteries is the mainstay. This type of battery has higher energy density and more and more convenient materials, but there are more Problems still need to be overcome. Lithium-sulfur batteries use sulfur as the positive electrode of the battery and metallic lithium as the negative electrode. Elemental sulfur has abundant reserves in the earth, and has the characteristics of low price and environmental friendliness. Lithium-sulfur batteries that use sulfur as a cathode material have a higher theoretical specific capacity of materials and a theoretical specific energy of batteries, reaching 1675m Ah / g and 2600Wh / kg, respectively, which is much higher than the widely used commercial ternary batteries.
And sulfur is an environmentally friendly element, which basically has no pollution to the environment, and is a very promising lithium battery; lithium metal batteries use lithium metal foil to replace graphite, which can accommodate more ions, but usually lithium Metal foils and electrolytes can cause adverse reactions, which can lead to overheating of the electrolyte and even combustion. This technology can reduce the volume of current lithium batteries by half. In theory, if the battery volume does not change, with a lithium metal battery installed The cruising range of electric vehicles will be doubled; lithium-air batteries are batteries that use lithium as the anode and oxygen in the air as the cathode reactant. Lithium-air batteries have a higher energy density than lithium-ion batteries because the cathode (mainly porous carbon) is very light, and oxygen is obtained from the environment without being stored in the battery. In theory, because oxygen is not a cathode reactant Limited, the battery's capacity depends only on the lithium electrode, and its specific energy is 5.21kWh / kg (including oxygen mass), or 11.4kWh / kg (excluding oxygen).
2. What are the basic requirements for energy carriers?
(1) The relative mass of atoms should be small;
(2) The electronic ability of gain and loss is stronger;
(3) The proportion of electron transfer is higher.
3. What are the main indicators of the battery?
(1) Capacity;
(2) Energy density;
(3) Charge and discharge rate;
(4) Voltage;
(5) Life span;
(6) Internal resistance;
(7) Self-discharge;
(8) Operating temperature range.

4. What are the characteristics of cathode materials (LFP, NCM, LiCo, etc.)?
(1) Higher redox reaction potential and high output voltage;
(2) High lithium element content and high energy density;
(3) Stable structure in chemical reaction;
(4) High conductivity;
(5) Good chemical stability and thermal stability, not easy to decompose and react;
(6) The price is cheap;
(7) The production process is relatively simple and suitable for large-scale production;
(8) Environmentally friendly and low pollution.

5. What are the characteristics of negative electrode materials (Li, C, AL, lithium titanate, etc.)?
(1) Layered structure or tunnel structure is conducive to de-embedding;
(2) Stable structure, good charge and discharge reversibility and cycle performance;
(3) Insert and deintercalate as many lithium ions as possible;
(4) Low oxidation-reduction potential;
(5) Low irreversible discharge capacity for the first time;
(6) Good compatibility with electrolyte solvents;
(7) Low price and easy access to materials;
(8) Good security;
(9) Environmentally friendly.
6. What are the ways to improve battery energy density?
(1) Increase the proportion of positive and negative active materials;
(2) Improve the specific capacity (gram capacity) of positive and negative materials;
(3) Lose weight and lose weight.
7. How to increase the charge-discharge rate of lithium-ion batteries?
(1) Improve the lithium ion diffusion capacity of positive and negative electrodes;
(2) Improve the ionic conductivity of the electrolyte;
(3) Reduce the internal resistance of the battery (ohmic internal resistance and polarization internal resistance).
8. What factors affect the cycle life of lithium-ion batteries?
(1) Deposition of negative metal lithium;
(2) Decomposition of cathode material;
(3) Formation and re-consumption of SEI;
(4) The influence of electrolyte is mainly manifested in: the total amount is reduced, impurities are present, water infiltration;
(5) The diaphragm is blocked or damaged;
(6) The positive and negative electrode materials fall off;
(7) External use factors.
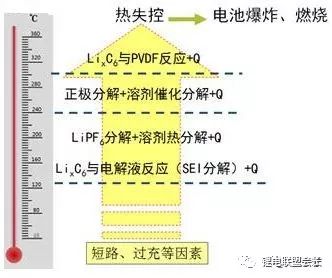
9. What is the decomposition temperature of the materials inside the lithium ion battery?
(1) SEI membrane decomposes, the electrolyte exothermic reaction, 130 ℃;
(2) Decomposition of electrolyte and heat generation, 130 ℃ -250 ℃;
(3) The decomposition of the cathode material generates a large amount of gas and oxygen, 180 ℃ -500 ℃;
(4) Reaction of binder and negative electrode active material, 240 ℃ -290 ℃.
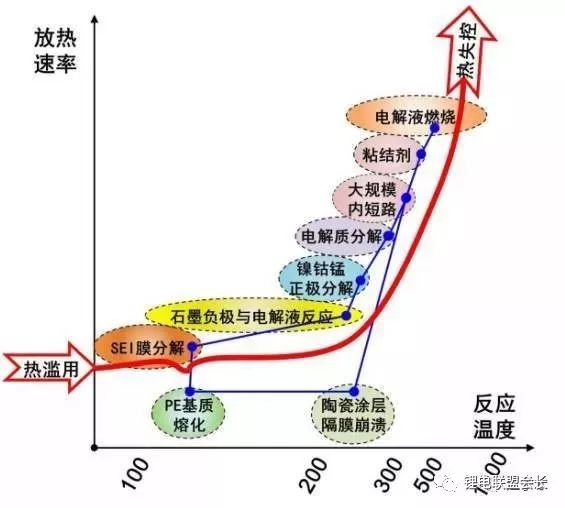
Generally, a process that generates a large amount of heat and gas due to short circuit caused by overcharge, large rate discharge, internal short circuit, external short circuit, vibration, collision, drop, impact, etc.
10. Several potential lithium battery materials in the future
(1) Silicon carbon composite negative electrode material, high energy density, industrialized above 400wh / kg, but serious volume expansion, poor circulation;
(2) Lithium titanate, more than 10,000 cycles, volume change <1%, no dendrite formation, excellent stability, fast charging, but high price, low energy density, about 170wh / kg;
(3) Graphene, which can be used for negative electrode materials and positive electrode additives, has excellent conductivity, fast ion transmission, poor first effect, about 65%, poor circulation, and high price;
(4) Lithium-rich manganese-based battery, energy density is about 900wh / kg, rich in raw materials, but the first effect is low, safety and cycle is poor, the rate performance is low;
(5) NCM ternary material, generally 250wh / kg, with silicon carbon anode, about 350wh / kg;
(6) CNTs, carbon nanotubes, with superior electrical conductivity and excellent thermal conductivity;

(7) Coated diaphragm, base film + PVDF + boehmite, improve the shrinkage resistance of the diaphragm, low thermal conductivity, prevent all thermal runaway;
(8) High voltage electrolyte, needless to say, as the energy density of the energy material, the voltage will increase accordingly;
(9) Water-based adhesives, out of environmental protection and health.
Pre-lithiation, before talking about this, let me first talk about the first-effect problems of half-cells (the positive electrode is the positive electrode material and the negative electrode is the metal lithium sheet) and the full battery.
This is the first effect of lithium cobalt oxide half-cells. If you do n’t understand that full-cell and half-cell are okay, you will understand that this is the first effect of cathode materials.
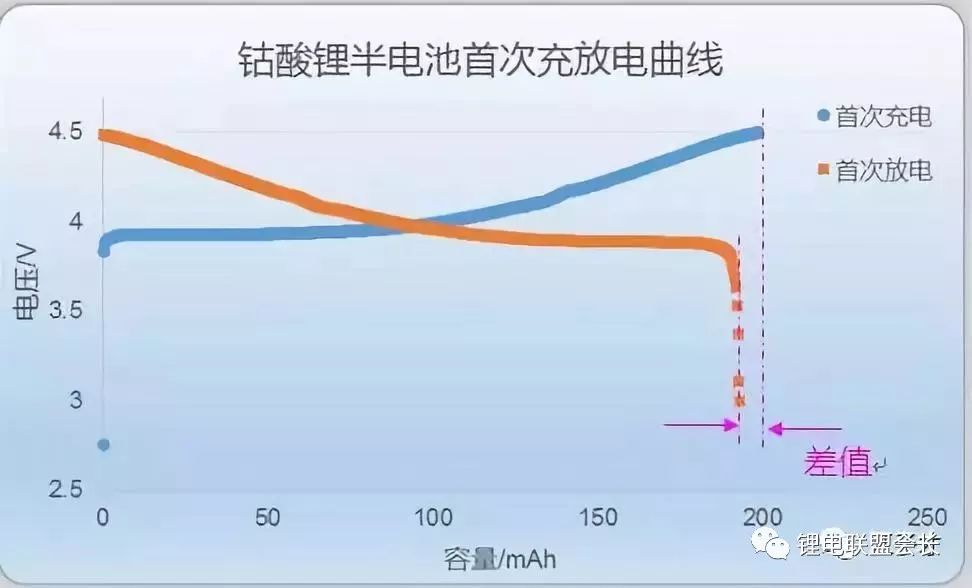
From the above figure, we can see that the first charge capacity of the half-cell is slightly higher than the first discharge capacity, that is to say, not 100% of the lithium ions released from the positive electrode during charging return to the positive electrode during discharge. The first discharge capacity / first charge capacity is the first efficiency of this half-cell.
The first effect of lithium iron phosphate positive half-cell
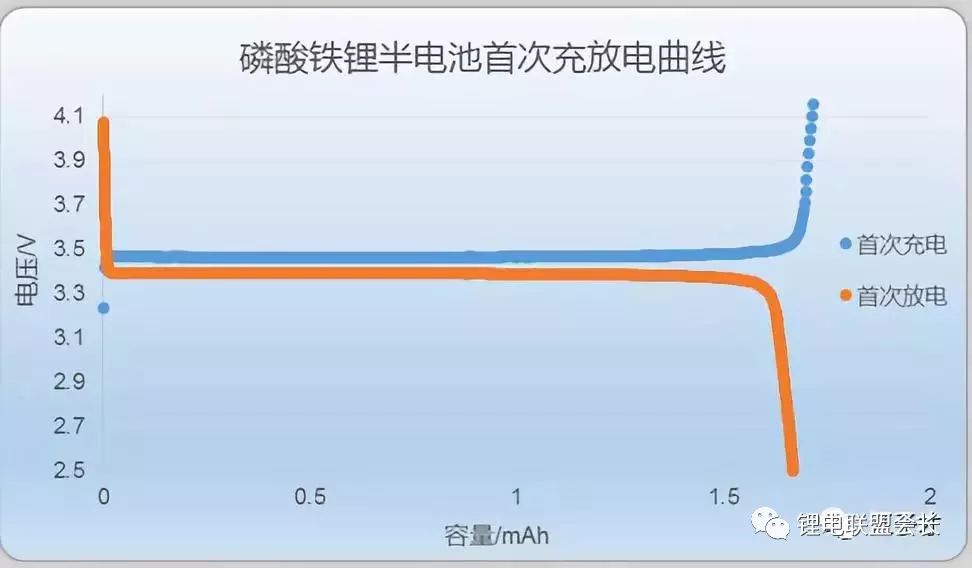
Ternary positive half-cell first effect

As can be seen from the above pictures, the first efficiency of ternary is the lowest, generally 85 ~ 88%; lithium cobaltate is second, generally 94 ~ 96%; lithium iron phosphate is slightly higher than lithium cobaltate, is 95% ~ 97%. The first effect of the positive electrode material is mainly due to the change in the structure of the positive electrode material after deintercalation occurs, there is not enough lithium intercalation position, and the lithium ions cannot be fully returned at the first discharge.
Graphite anode half-cell first effect

The difference between a graphite battery half-cell and a positive electrode is that graphite is used as the positive electrode and the metal lithium sheet is used as the negative electrode. Therefore, the first effect of graphite is significantly lower than that of the positive electrode material. The main reason is that lithium ions pass through the electrolyte and will A SEI film is formed on the graphite surface, which consumes a lot of lithium ions. The lithium ions dedicated to the SEI film cannot return to the negative electrode.
The first efficiency of the whole battery. After the battery is filled, it needs to undergo the process of formation (charging only) and volume division (with charge and discharge). Generally speaking, the first step of formation and volume division is the charging process. And, it is the first full battery charge capacity; the second step of the capacity dividing step is generally to discharge from a fully charged state to empty power, so this step capacity is the discharge capacity of the full battery. Combining the two, we get the algorithm of the first efficiency of the full battery:
Full battery first efficiency = Discharge capacity second step discharge capacity / (Formation charge capacity + Divide capacity first charge capacity)
In order to reduce the deviation in the daily life, the second full discharge capacity is taken as the battery capacity.
In summary, we can draw a conclusion. If the battery positive electrode uses a ternary material with an initial efficiency of 88%, and the negative electrode uses a graphite material with an initial efficiency of 92%. For this full battery, the first efficiency is 88%, that is, when the first effect of the positive electrode is 88% and the first effect of the negative electrode is 92%, the first effect of the full battery is 88%, which is equal to the lower positive electrode.
Of course, in addition to the battery material affecting the first effect, the specific surface area of ​​the electrode material is also an important factor. The larger the specific surface area of ​​graphite, the larger the SEI film formed, the more lithium ions are consumed, and the lower the first effect. In addition, it is also related to the formation and charging system of the battery. Charging the appropriate SOC will also affect the first effect of the battery to a certain extent.
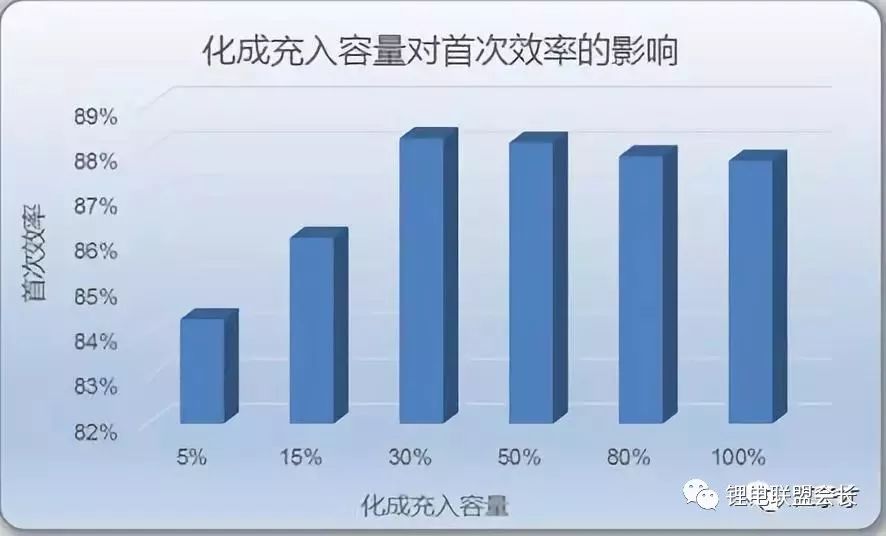
For a full battery, the SEI film formed at the negative electrode interface at the time of formation consumes lithium ions released from the positive electrode and reduces the capacity of the battery. If we can find a lithium source from the outside of the cathode material, let the formation of the SEI film consume lithium ions from the external lithium source, so as to ensure that the lithium ions released from the cathode will not be wasted in the formation process, and finally the whole battery can be improved. capacity. This process of providing external lithium sources is pre-lithiation.
Below I will borrow an article to tell you about the main pre-lithiation method, but I have only seen one method, which is the method of spraying lithium powder on the negative electrode.
1. The negative electrode formation method in advance
We can let the negative electrode be formed separately, and then assemble it with the positive electrode after forming the SEI film on the negative electrode, so as to avoid the loss of the lithium ion of the positive electrode and greatly improve the first efficiency and capacity of the whole battery, as shown in the schematic diagram:
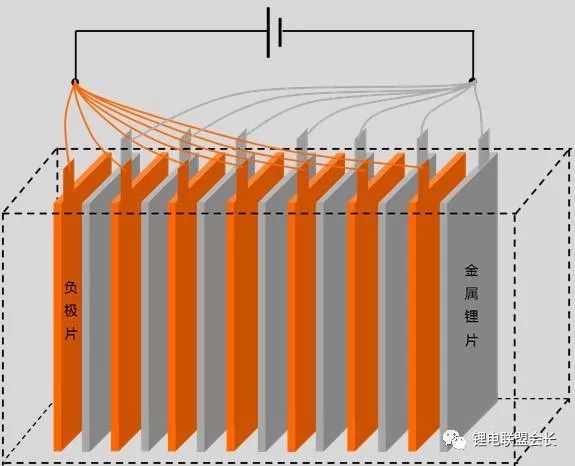
In the picture above, the negative and lithium plates are immersed in the electrolyte and charged by an external circuit. In this way, it can be ensured that the lithium ions consumed at the time of formation come from the metal lithium sheet instead of the positive electrode. After the formation of the negative electrode sheet is completed, it is assembled with the positive electrode sheet again, and the cell no longer needs to be formed, so that the lithium ion of the positive electrode will not be lost due to the formation of the SEI film of the negative electrode, and the capacity will be significantly improved.
The advantage of this pre-lithiation method is that it can simulate the normalization process to the maximum extent, and at the same time ensure that the formation effect of the SEI film is similar to that of the full battery. However, the two processes of the advance formation of the negative electrode sheet and the assembly of the positive and negative electrode sheets are too difficult to operate.
2. Negative electrode spraying lithium powder method
Since it is difficult to use the negative electrode sheet to convert to lithium supplementation alone, people think of a method of spraying lithium powder directly on the negative electrode segment. First, a stable metal lithium powder particle must be produced. The inner layer of the particle is metal lithium, and the outer layer is a protective layer with good lithium ion conductivity and electron conductivity. In the pre-lithiation process, the lithium powder is first dispersed in an organic solvent, and then the dispersion is sprayed on the negative electrode sheet, and then the residual organic solvent on the negative electrode sheet is dried, so that the pre-lithiated negative electrode sheet is obtained. Subsequent assembly work is consistent with the normal process.
During the formation, the lithium powder sprayed on the negative electrode will be consumed in the formation of the SEI film, thereby maximally retaining the lithium ions extracted from the positive electrode and increasing the capacity of the entire battery.
The following figure is a comparison chart of the efficiency of the negative electrode silicon alloy and the positive electrode lithium cobalt oxide full battery. It can be seen that after the pre-lithiation of the negative electrode, the efficiency has been significantly improved for the first time:
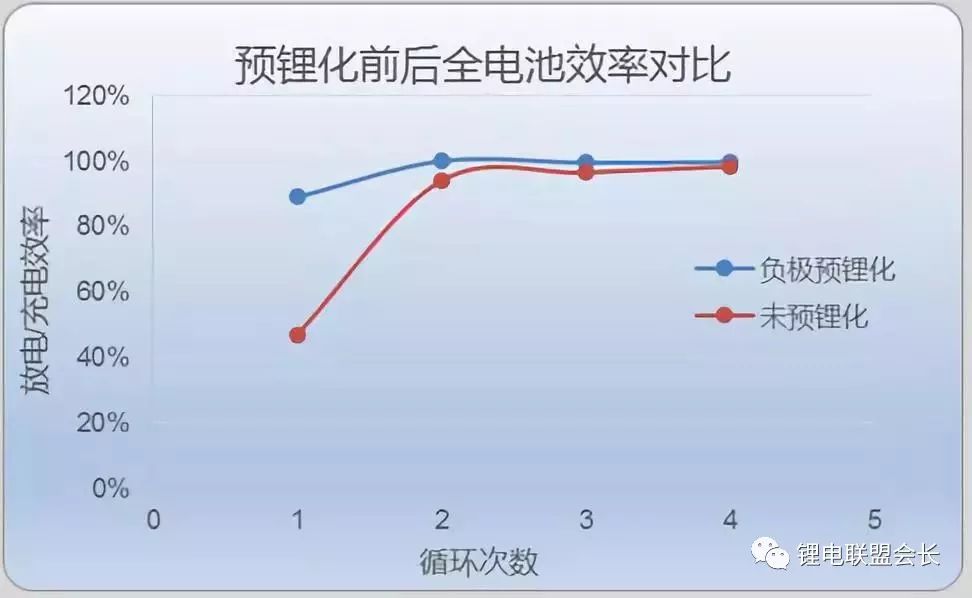
The disadvantage of using this pre-lithiation method is that the safety is more difficult to guarantee, and the cost of material and equipment transformation is higher.
3. Negative three-layer electrode method
Due to the limitations of equipment and technology, simple high-cost transformation for pre-lithiation is not a preferred choice for battery factories. If pre-lithography can be completed in a manner familiar to battery factories, the promotion is greatly enhanced. The three-layer electrode method described below is simpler for battery manufacturers. The core of the three-layer electrode method is the treatment of copper foil. The schematic diagram of copper foil is as follows:
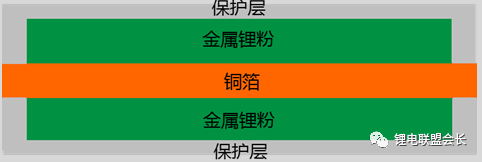
Compared with the normal copper foil, the copper foil of the three-layer electrode method is coated with the metal lithium powder required for later conversion. To protect the lithium powder from reacting with air, a protective layer is coated; the negative electrode is directly coated on the On the protective layer. The overall schematic diagram of the single-layer electrode after assembly is as follows:

After the cell is filled with liquid, the protective layer will be dissolved in the electrolyte, so that the metal lithium contacts the negative electrode, and the lithium ions consumed to form the SEI film during the formation are supplemented by the metal lithium powder. The icon of the electrode after charging is as follows: this method does not have strict requirements on the processing conditions of the battery factory, but the stability of the protective layer at the pole piece winding, rolling, cutting and other stations is a great challenge to the development of electrode materials It is also difficult to ensure the adhesion of the negative electrode material after the lithium metal powder has disappeared.
4. The positive electrode lithium-rich material method
The friends who work in the enterprise must have deeply experienced: even if something that can be successful under laboratory conditions, it may be difficult to move to large-scale production in the enterprise. The cost of equipment transformation, the cost of batch input of materials, and the cost of controlling the processing environment, etc. may all become fatal injuries that the new technology cannot promote. For the industry where lithium battery technology and equipment are basically mature, the pre-lithography scheme preferred by enterprises will definitely be a method that can be directly promoted without making too many on-site changes or even taking it over. The positive electrode lithium-rich material method just meets the needs of battery manufacturers in this regard.
The so-called positive electrode lithium-rich method can be simply understood as that there is such a material that when it is formed, the number of lithium ions released by her positive electrode is several times that of the materials currently used. . When the first effect of the negative electrode is lower than that of the positive electrode, too much lithium ions will be lost to the negative electrode at the time of formation, so that the effective space of the positive electrode cannot be underfilled by lithium ions after discharge, resulting in a waste of lithium intercalated space of the positive electrode. If a small amount of high-gram capacity lithium-rich material is added to the positive electrode, this can provide more lithium ions for the formation of the SEI film at the time of formation, and there is no need to worry that the lithium-rich material will not be able to intercalate lithium again during discharge (because All lithium ions provided by lithium-rich materials are consumed).
The various pre-lithiation methods described above are aimed at full batteries with a negative electrode that has a lower first-effect than the positive electrode. After full-cell pre-lithiation, the highest efficiency for the first time can only reach the level of a half-cell positive electrode material. For batteries with lower first-efficiency of the positive electrode, the above method is basically useless, because the first-effect of the full battery is limited by the fact that the positive electrode no longer has enough space for lithium insertion after charging, even if the external lithium is supplemented, it cannot The positive electrode is embedded, so it has no effect.
customized high voltage,High voltage ESS, HV battery, large scale battery; one-stop solution provider for energy storage; high voltage battery, high voltage battery storage system, high voltage battery cabinet
Shenzhen Enershare Technology Co.,Ltd , https://www.enersharepower.com