This article is selected from "Effect of Induced Metal Contaminants on Lithium-ion Cell Safety", here is an experiment with a 1.4Ah multilayer soft package sample.
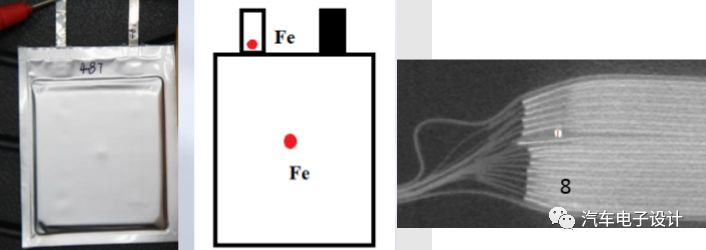
Research problem definition and experimental verification
Due to the possibility of internal short circuits during use, metal particles in lithium-ion batteries may pose safety risks. Different external abuse experiments and real metal particle residues may have different problems. Therefore, to evaluate how metal particles cause battery short-circuits, the following problems need to be solved:
1) Does the severity of the short circuit depend on the location of the particles in the battery?
2) Does the severity of the short circuit depend on the size of the particles? (Four different forms of internal short circuit caused by diaphragm penetration and dendrite formation)
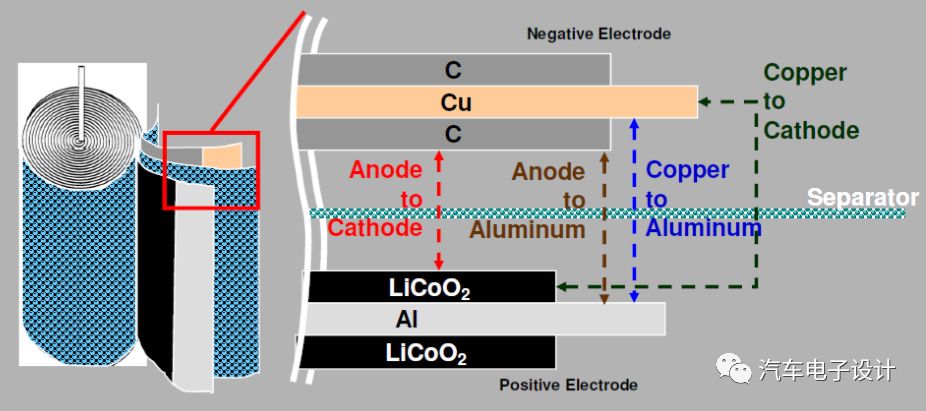
Heat production power and curve under different internal short circuit forms
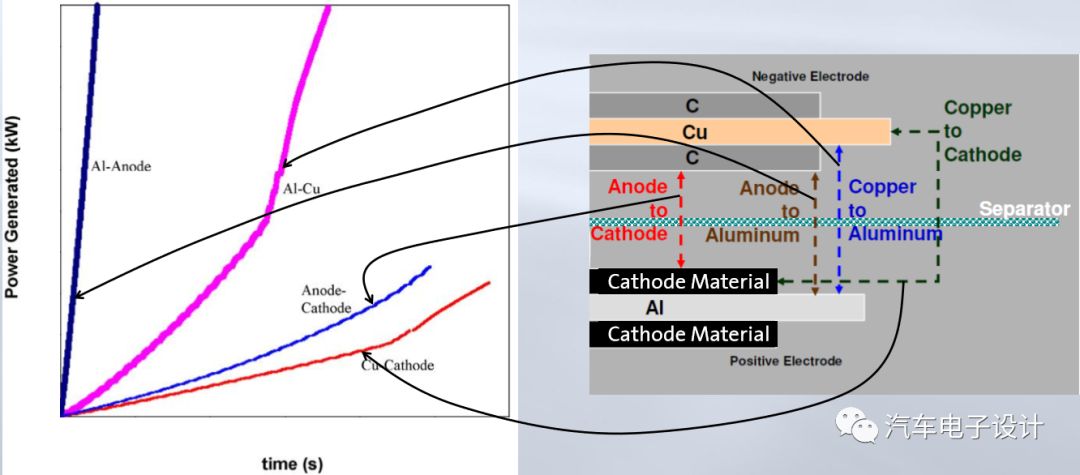
Under different particle sizes, how to detect the impact caused by metal particles and reject the battery during the manufacturing process?
3) Does the severity of internal short circuit increase with cycling or storage?
Experimental method
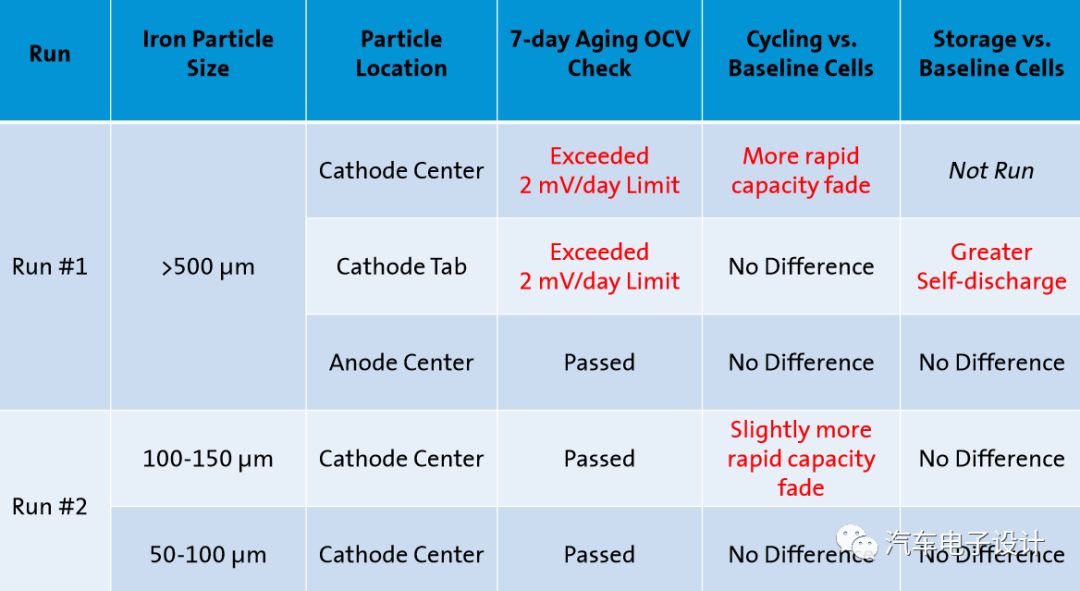
Test 1: 500-700μm iron particles (easy to handle)
Test 2: 50-150μm iron particles (minimum size of known metal contaminants)
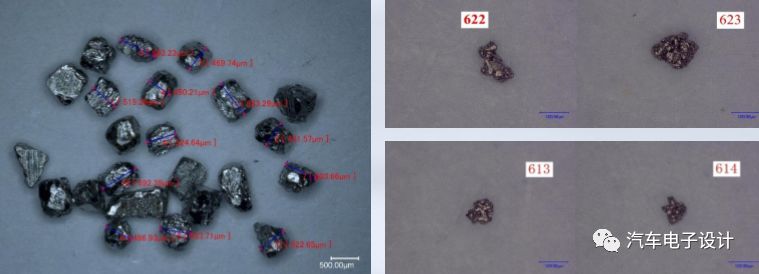
Detection method and test
Hi-Pot test after electrode stack assembly
This experiment has a question of confidence. Larger particles can be detected, and the test results will be different only on the cathode surface.
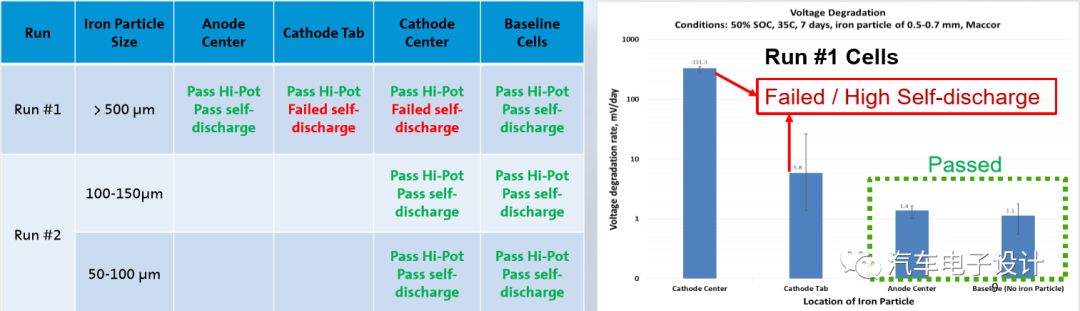
Self-discharge (Delta-OCV) is checked during the aging process (50% SOC, 7 days at 35ºC)
Cycle life test (100% DOD, 1C / 1C rate, 35ºC under battery pressure)
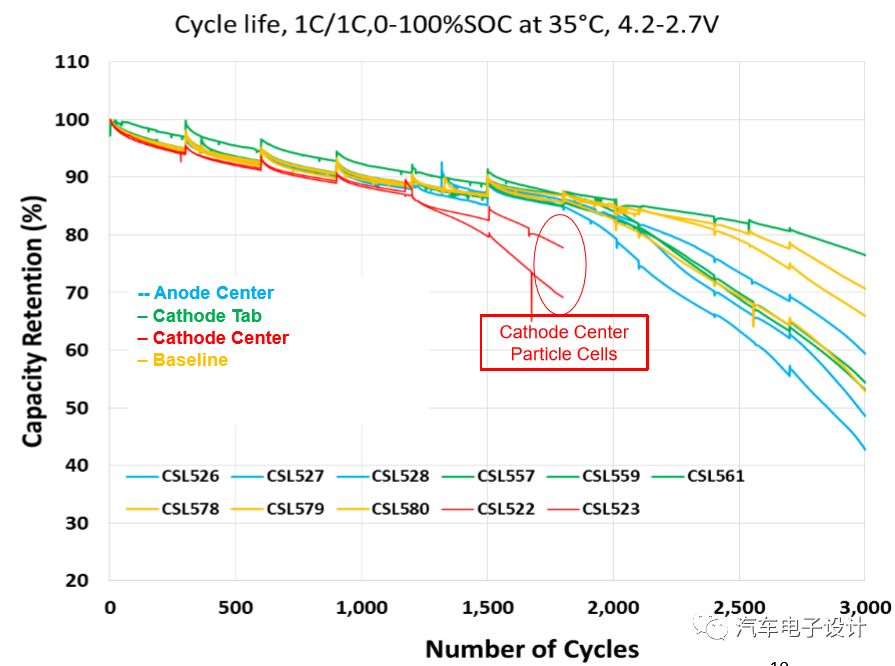
The experimental results under different cycles are most obvious at the center of the positive electrode, which makes the capacity decay faster.
Larger metal particles
Smaller size metal particles
Storage test (100% SOC under 35ºC pressure)
Using the self-discharge test, the metal particles on the positive pole piece will produce a large voltage drop, and the actual particles have little difference in the attenuation of the storage capacity.
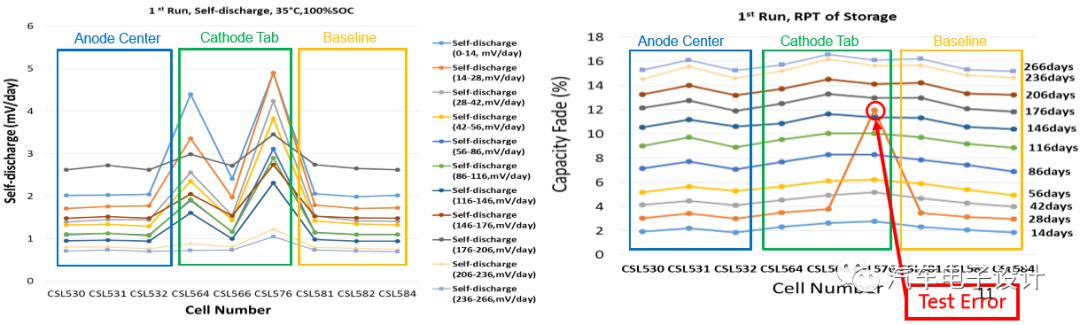
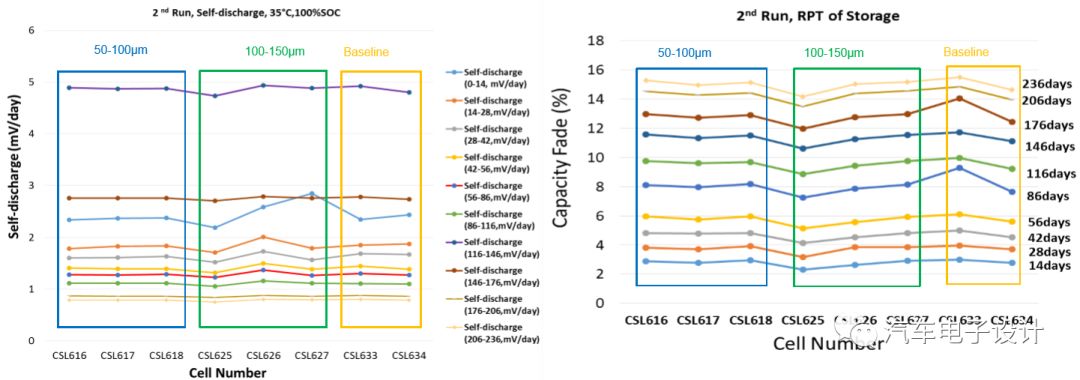
After cycling and storage, the self-discharge speed of the battery cell is still faster
We detected large discharge batteries. The metal contaminants are more obvious in the center of the cathode. This cycle life will obviously accelerate and jump down later.
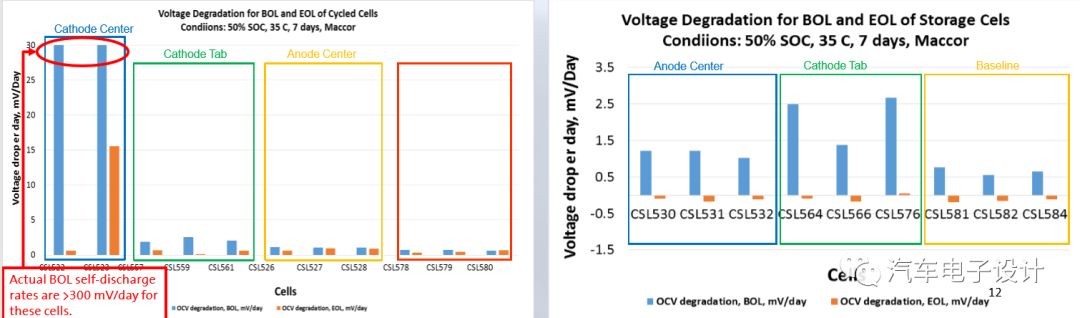
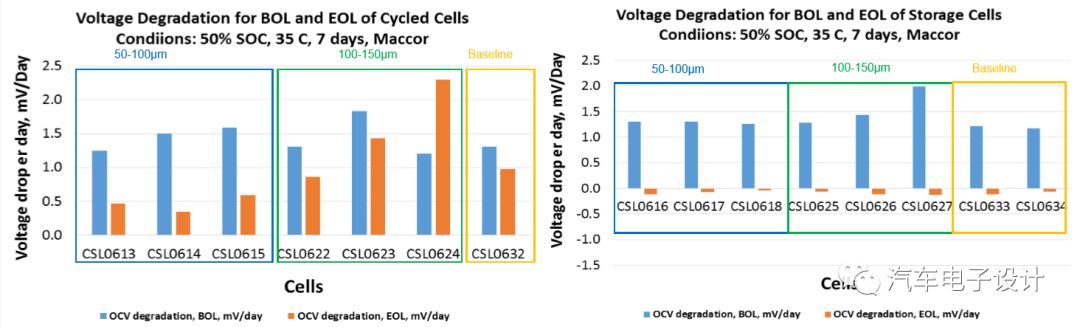
Conclusion of the small battery experiment:
Even particles much larger than the thickness of the diaphragm (20-28 times) will not produce internal short circuits. The experimental conditions are cycle under pressure
The metal particles of the anode will not cause the internal battery to short circuit
Only the metal particles initially located on the cathode, if the size and quality are sufficient, will cause the internal battery to short circuit
In the manufacturer’s aging/storage process steps, large metal particles can be detected through self-discharge and capacity loss
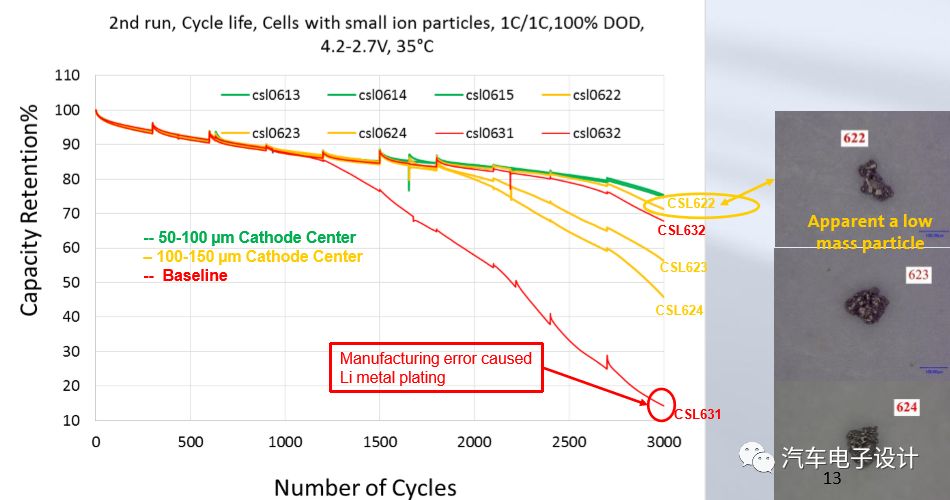
Particles below 100μm have a limited impact, while particles near 150μm will indeed cause cell cycle life failure
Some thoughts: Without external abuse, if there are metal particles in the process, the results are controllable. It is okay to use the cycle and calendar to store the results of the experiment, and the battery does not have safety problems. There is also the self-discharge experiment, which is the most effective method for us to detect new batteries, which can effectively detect the batteries that have dived later
Power X (Qingdao) Energy Technology Co., Ltd. , https://www.solarpowerxx.com